Non-ferrous smelting is mainly divided into the following major areas: aluminum smelting, copper smelting, lead smelting, zinc smelting, nickel smelting, gold and silver. The editor will introduce these refractory materials for non-ferrous smelting one by one, combined with our company’s product introduction.
In the world’s non-ferrous metal production, the annual output of aluminum ranks first, far exceeding other non-ferrous metals. The aluminum industry consumes much more refractory materials every year than the total amount of refractory materials consumed by copper, lead, and zinc smelting. The production method of metallic aluminum is a fixed two-step production method: in the first step, alumina is produced from bauxite ore by wet method; in the second step, industrial alumina is used as raw material and metal is produced by molten salt electrolysis. aluminum. High-temperature kilns used in the production process include rotary kilns, molten salt electrolyzers, aluminum melting furnaces, etc.
Aluminum industrial furnaces consume a lot of refractory materials. The reason is that when producing Al2O3, the alkaline substances in the materials corrode the refractory materials of the rotary kiln particularly seriously. During the process of smelting aluminum, even at lower temperatures, metallic aluminum still has strong penetrating ability. Once it penetrates into the bricks, it will react with the SiO2 in the bricks, reduce Si, and destroy the structure of the refractory material. The furnace lining will produce a deteriorated layer, which will become loose, peeled off and damaged. The reaction: 3SiO2 + 4A1 —2A12O3 + 3SiO2
Therefore, refractory materials containing SiO2 are not suitable as furnace building materials for aluminum metal smelting equipment. Therefore, generally aluminum industrial furnaces use refractory materials. In addition to high alumina bricks, carbonaceous products are commonly used.
Refractory materials for alumina rotary kiln
At present, due to raw materials, most of the alumina production processes in my country adopt the sintering method and the combined method. Rotary kilns are mostly used for the drying and tender firing of bauxite and the roasting of aluminum hydroxide. In recent years, the introduced fluidized roasting device has been widely used, but in some old factories, rotary kilns still account for a large proportion.
The rotary kiln is a sintering kiln for alumina clinker. When making alumina, first put the bauxite, soda ash and lime into the rotary kiln in proportion, burn it at 1200~1300℃ and then leave the kiln, and then properly process it to make aluminum hydroxide and mother liquor; It is put into a rotary kiln and fired at a high temperature of 1200℃ to make it. The calcining process in the rotary kiln is: the high-temperature flame and the heated material move in reverse directions in the furnace. Soda lime aluminum phosphate slurry (water content 40%) or aluminum hydroxide (water content 12% ~ 18%) is added from the end of the kiln. After low-temperature drying, dehydration, heating, and high-temperature calcination, the material is discharged from the kiln head and the high-temperature gas is discharged from the kiln head. flows towards the end of the kiln. Therefore, the kiln is divided into a preheating zone and a high-temperature calcining zone. In order to prevent the slurry from sticking to the kiln lining and enhance the heat transfer process during the heating and calcining process, chains are also set up between the refractory masonry bodies. During the rotation of the kiln body, they continuously hit the materials and lining bricks, thus damaging the kiln lining. has a certain impact on its service life.
The kiln body of the rotary kiln for alumina production is a cylinder made of welded steel plates and lined with refractory materials. Refractory materials work in harsh environments and harsh conditions. It should have the following characteristics: strong resistance to alkali erosion; able to work at high temperatures of 1200~1300°C for a long time without being damaged; able to withstand the impact of dynamic loads; able to resist the erosion of furnace materials; able to withstand the erosion of high-temperature airflow. The refractory materials used in the rotary kiln are mainly high alumina bricks and magnesia chrome bricks, while the low-temperature drying kiln is lined with clay bricks. The refractory materials for rotary kilns used in alumina production are shown in the table below.
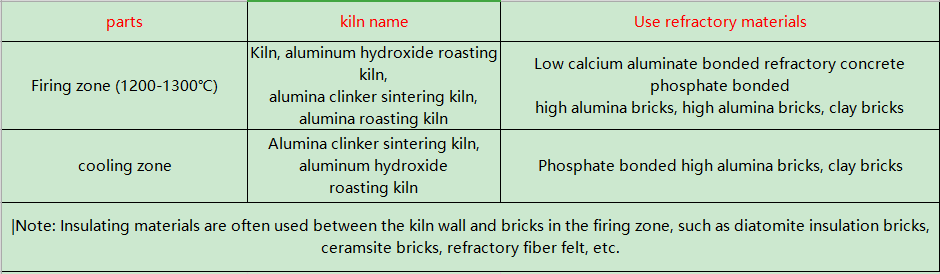
Nowadays, amorphous refractory materials have been widely used in the aluminum industry. The kiln mouth of the rotary kiln is easily deformed and damaged due to the high temperature wear and thermal shock stress of the material; in the transition zone of the alumina clinker kiln , the ambient temperature is 400~1000℃, it is seriously affected by alkali corrosion and mechanical damage (vibration, distortion), and the lining often falls off. Rotary kilns also use steel fiber reinforced castables, which are mainly used in preheating zones, kiln mouths, kiln tails, and coolers.
Aluminum hydroxide roasting is the last process in the alumina production process. It mainly dries the attached water and crystal water in the aluminum hydroxide filter cake, and converts a part of β-type alumina into a-type alumina. At present, the aluminum hydroxide roasting of major domestic alumina manufacturers has fully or partially adopted the introduced fluidized roasting device. Fluidized roasting equipment is divided into three types: fluidized flash roasting furnace, circulating fluidized bed roasting furnace, and suspension roasting furnace. Although the refractory materials used are different, a large number of amorphous refractory materials (refractory plastics) are used. or refractory castable), its dosage accounts for 50% to 70% of the refractory materials used. The amorphous refractory materials of the fluidized flash roasting furnace are all imported from Germany, and the refractory materials of the circulating fluidized bed roasting furnace are all produced domestically.
The alumina gas suspension roasting furnace is a special equipment used for roasting aluminum hydroxide. Its technology and automation level are very high. The roasting process is completed at the operating temperature of the high-temperature furnace body at about 1200°C and at high speed. At the same time, due to the processed Alumina materials have high hardness and good fluidity, and have very strict requirements on the quality of alumina products. Any impurities mixed into the lining materials will directly affect the performance of the products. Therefore, the refractory materials must meet the following conditions: high temperature resistance, Wear, high strength, good thermal stability, good integrity, and strong sealing.
The electrolytic cell is the core equipment for the production of electrolytic aluminum. The electrolytic cell is usually a rectangular steel shell lined with carbon bricks. There is a carbon anode suspended in the electrolytic cell, and the bottom of the carbon cell is the cathode. Aluminum electrolysis uses cryolite, aluminum fluoride, lithium fluoride and other molten liquids as electrolytes. Al2O3 is melted at about 970°C and ionized under the action of electric field force. The metal aluminum melt restored by electrolysis is deposited on the cathode and anode at the bottom of the tank. The released oxygen reacts with the carbon anode to generate CO2 or CO. The heat released by the electrochemical reaction keeps the electrolytic tank and aluminum in a molten state. Liquid aluminum is released from the tank at certain intervals, and a certain amount of alumina and cryolite are added to the tank; the electrolysis temperature is 900~1000°C.
The working layer at the bottom of the electrolytic cell is generally built with carbon blocks. However, due to the reaction of carbon and sodium to form new compounds, the brick lining structure is loose, the strength is reduced, and cracks appear in the carbon blocks. Then the electrolyte and aluminum liquid enter along the crack, and the aluminum reacts with the carbon at high temperature to form a loose bond with the carbon, which causes the crack to expand, eventually leading to deformation of the electrolytic cell shell and severe corrosion of the lining, shortening the service life. Therefore, the cathode material at the bottom of the electrolytic cell is being changed from the original amorphous carbon brick to semi-graphitized carbon brick or graphitized carbon brick.
The main causes of damage to the side wall lining of the aluminum electrolytic cell are: oxidation of the material caused by air inhalation between the steel shell and the brick lining; corrosion of cryolite, NaF and aluminum liquid at high temperatures; erosion caused by melt flow; temperature fluctuations and thermal expansion Cause thermal stress.
The side walls of aluminum electrolytic cells have always been made of amorphous carbon blocks, graphite carbon blocks, etc. The most fatal shortcomings of this type of material are poor anti-oxidation performance and low strength. In order to prevent the sidewalls from oxidation and have greater resistance, the sidewalls are developing in the direction of partially or entirely using SiC materials. Silicon carbide bricks bonded with silicon nitride are best used. Silicon nitride-bonded silicon carbide bricks have excellent high-temperature mechanical properties and good thermal conductivity, and are easy to form condensation slag on the inside; they have high resistivity, reducing current loss on the side walls; the material is not easily oxidized; it does not interact with liquid aluminum and ice crystals It reacts with melts such as stone; it has high mechanical strength, can also greatly reduce the thickness of lining bricks, increase the volume of electrolytic cells, and stabilize operation. For example, when carbon bricks were originally used, the side wall thickness was about 200~400 mm, but when silicon nitride-bonded silicon carbide bricks were used, the side wall thickness was only 75 mm.
Barrier layer under the tank bottom
In the production of electrolytic aluminum, the vapor and liquid of Na and NaF can enter the heat insulation layer below through the cathode material at the bottom of the tank. When NaF or the like enters the heat insulation layer, the thermal conductivity increases, the thermal efficiency of the electrolytic cell decreases, and the operating conditions deteriorate until the cell is damaged. The “barrier layer” under the cathode material is a layer of material sandwiched between the cathode refractory material and the thermal insulation material that can prevent electrolyte penetration and has excellent thermal insulation properties. A new type of “barrier” material, dry anti-seepage material, is now in good use.
Refractory materials for refining furnaces and holding furnaces
The commonly used smelting furnaces for the melting and alloying of primary aluminum ingots and scrap aluminum are mostly fixed or tilting reverberatory furnaces with gas or oil, and some also use resistance reverberatory furnaces and induction spiral furnaces. Although the temperature of aluminum liquid and aluminum alloying in the smelting furnace is only 700~800°C, magnesium, silicon and aluminum in aluminum and aluminum alloys are very active and can easily react with some components in refractory materials to form refractory materials. damage. The corrosion damage mechanism of aluminum smelting furnaces is mainly as follows: liquid aluminum easily enters refractory materials;
The alloying elements in aluminum and its alloys have a strong ability to restore some oxides, and the redox reaction produced is a strong exothermic reaction; some alloying elements such as magnesium have high vapor pressure, and their vapor is easier to regenerate than liquid aluminum. Entering the refractory material, and then being oxidized after entering the refractory material, eventually leading to the deterioration of the refractory material, loose structure and damage;
During the smelting process of large-scale aluminum melting furnaces, due to the continuous addition of aluminum ingots and alloys, the impact and wear of aluminum ingots and alloy blocks on the furnace mouth, furnace bottom and furnace walls are very serious;
The addition of aluminum ingots and alloy blocks, the outflow of liquid aluminum, fluctuations in temperature in the furnace, etc., can cause thermal shock damage to the refractory lining.
Refractory materials for aluminum smelting reverberatory furnaces are required to be resistant to the entry of liquid aluminum and magnesium vapor, and have excellent resistance to wear and thermal shock. The lining of the reverberatory furnace for aluminum smelting that touches the aluminum liquid is generally built with high-aluminum bricks with an Al2O3 content of 80% to 85%; when smelting high-purity metallic aluminum, mullite bricks or corundum bricks are used. Silicon carbide bricks bonded with silicon nitride are used in areas prone to corrosion and wear, such as hearth slopes and used aluminum materials. Parts such as aluminum flow troughs and aluminum outlets are severely eroded by liquid aluminum. Generally, self-bonding or silicon nitride-bonded silicon carbide bricks are used, and staggered quartz bricks are also used as linings. As the aluminum outlet blocker, it is better to use vacuum cast refractory fiber. Furnace linings that do not come into contact with liquid aluminum generally use clay bricks, clay refractory castables or refractory plastics. The lining of the aluminum flow tank is generally made of silicon carbide bricks, or prefabricated fused foam silicon bricks can be used.
Now, with the enlargement of aluminum melting furnaces and the requirements for intensified tempering, high-strength aluminum penetration-resistant castables have been well received due to their excellent resistance to the entry of aluminum liquid and magnesium vapor, as well as their excellent anti-wear and thermal shock resistance. application.

